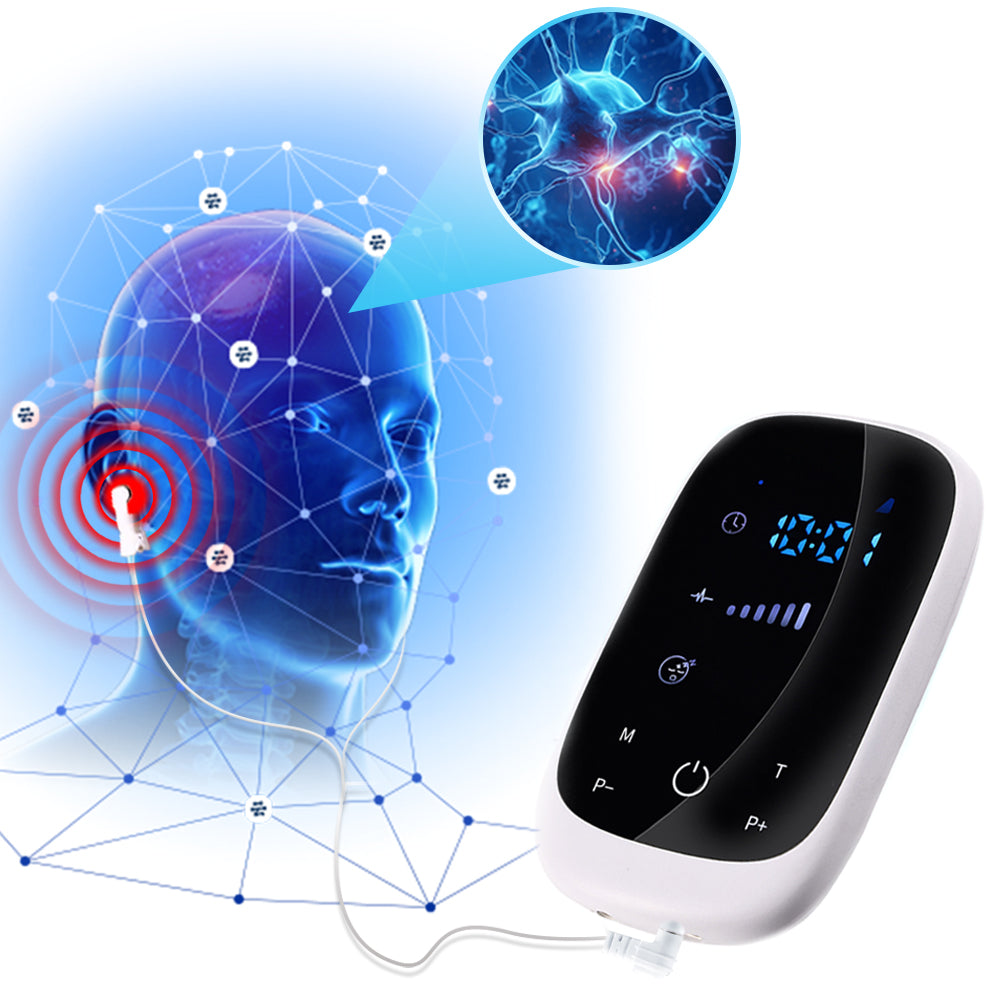
Cranial Electrotherapy Stimulation- CES Therapy
Share
The CES Insomnia Sleep Aid Device appears to be a Cranial Electrotherapy Stimulation (CES) device, a non-invasive, FDA-cleared technology used for anxiety, depression, insomnia, and pain management. Here’s a breakdown of its potential benefits, scientific backing, and considerations for wellness providers:
------
What is CES (Cranial Electrotherapy Stimulation)?
CES delivers low-level electrical currents (microamps) to the brain via ear clips or electrodes, modulating neural activity to promote relaxation and improve sleep.
Key Features of This Device:
✅ Non-invasive & Drug-Free – No side effects or dependency risks.
✅ Smart Touchscreen Interface – User-friendly controls for personalized therapy.
✅ Supports Hormone Balance – May influence serotonin, melatonin, and GABA.
✅ Multi-Condition Relief – Targets insomnia, anxiety, depression, migraines, and chronic pain.
✅ Wellness Clinic Integration – Ideal for sleep centers, holistic practitioners, and mental health providers.
------
Scientific Support for CES
• Insomnia & Sleep Disorders:
◦ Studies show CES improves sleep quality by regulating brainwave activity (e.g., increasing alpha waves for relaxation).
◦ A 2019 meta-analysis found CES effective for chronic insomnia with minimal side effects (Journal of Clinical Sleep Medicine).
• Anxiety & Depression:
◦ FDA-cleared for anxiety, depression, and PTSD.
◦ Research suggests CES boosts serotonin and endorphins, aiding mood regulation (Psychiatric Clinics of North America).
• Chronic Pain & Migraines:
◦ CES may reduce pain perception by modulating the limbic system (Pain Research and Management).
------
Why This Device Could Revolutionize Sleep & Wellness Care?
1. Alternative to Medications – Safe for patients avoiding sedatives or SSRIs.
2. Holistic Integration – Complements therapies like CBT-I (Cognitive Behavioral Therapy for Insomnia), meditation, and acupuncture.
3. Scalable for Clinics – Can be offered as an in-office treatment or prescribed for home use.
4. Preventive Health – Supports stress resilience and circadian rhythm alignment.
------
Considerations for Providers
• FDA Clearance: Ensure the device is FDA-cleared (if marketed in the U.S.) or meets regional regulatory standards.
• Patient Suitability: Best for mild-to-moderate insomnia/anxiety; severe cases may need additional interventions.
• Protocols & Training: Staff should be trained on proper usage and contraindications (e.g., epilepsy, pacemakers).
------
Marketing & Implementation Tips
• Target Audience:
◦ Insomnia sufferers, chronic pain patients, stressed professionals, and holistic health seekers.
• Clinic Integration:
◦ Offer free trials or demo sessions to build trust.
◦ Bundle with sleep hygiene coaching or stress management programs.
• Digital Outreach:
◦ Highlight drug-free benefits on social media (e.g., "No pills, just peace").
◦ Share testimonials from patients who reduced medication use.